Erin Spicer, Staff Writer |
This past week, Biogen, a pharmaceutical drug company, released a statement saying they requested the Food and Drug Administration approve their new drug, aducanumab, to treat people with the earliest signs of Alzheimer’s disease and mild cognitive impairment. If approved, over 10 million Americans could qualify for treatment. However, the most recent data analyses have not been released, and most experts are unsure of how well the drug actually works. The company has stated that their drug does not cure or prevent Alzheimer’s and they claim it may only slow cognitive decline in some patients.
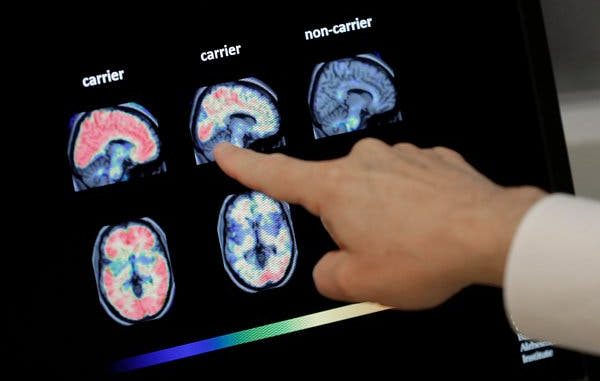
Aducanumab is a monoclonal antibody which attaches to specific proteins in order to disable them. The drug is used to target a key protein in Alzheimer’s disease, called beta amyloid, which accumulates in plaque in patients’ brains. Initial research with the drug showed little difference in patients, however, after raising the dose the drug started to show results. Aducanumab is given to patients via injection once a month with the hope that patients treated early in the course of their disease may recover or the progress of the disease may be slowed.
Early studies had approximately 1,600 patients, and the brain scans of those patients showed a reduced amount of beta amyloid as a result of the drug. In one of the studies, cognitive decline slowed, and in another, the patients experienced no benefit. Even after Biogen altered the study to raise the dosage, the results remained the same. Without the latest results from their research, it may be rather difficult for Biogen to get approval for aducanumab. Many experts question the legitimacy of the drug’s effectiveness because of Biogen’s failure to publish its research in a peer reviewed journal. The company plans to present their findings formally at a scientific meeting this December. This presentation will weigh in heavily on whether the drug will be granted approval. Those in attendance will pay close attention to the changes made mid-study to the doses.
Regardless of how well the drug works, or if it does at all, what’s important is that the medical community continues to strive for the answer to a disease that affects approximately 5.5 million Americans and 44 million people worldwide. Organizations such as The Alzheimer’s Association are non-profit organizations that helps fund research to fight Alzheimer’s. For more information about how to help stop Alzheimer’s visit act.alz.org.
My mother was in the 3rd phase study if Aducanumab for 2 1/2 years up until it was cancelled earlier this year. My family and I were shocked that the study was being canceled because we were so amazed at the lack of progression of my mother’s disease during the time that she was receiving it. I also read other testimonials from patients who were also in the study after it was canceled saying the exact same thing. It did not sit well with me or my family that the study had been canceled when we personally saw such encouraging results. My mother’s disease has progressed since stopping the drug in February. When I heard that Biogen was reconsidering their findings after a closer look at a larger data set, I was elated and, quite frankly, not surprised because it confirmed our personal feelings about the drug’s effectiveness. I would’ve appreciated it if the person who wrote this article would’ve considered some of the actual personal experiences of the people who were involved in the study.